
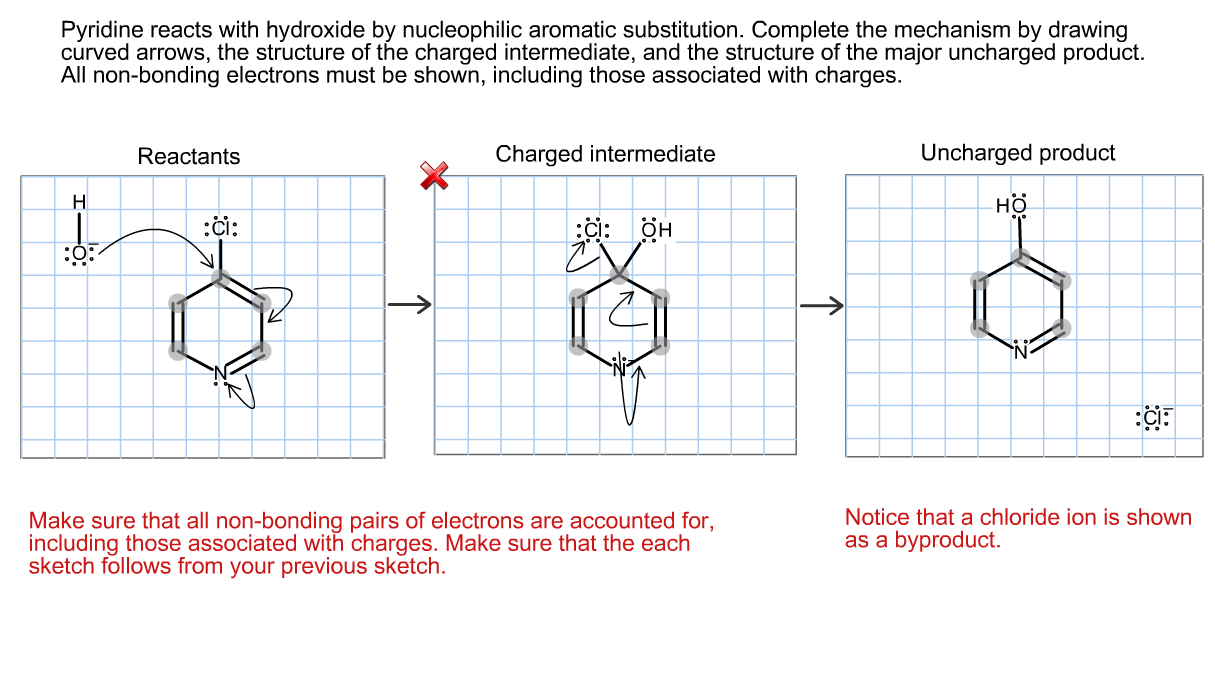
Ropentafluorobenzene with nucleophilic reagents. p-substituted products were ~ b t a i n e d "by N, N-disubstituted pentafluorophenylamine were first prepared' by reaction of perfluo~ reaction of chlorobenzene with secondary amine. Were calculated from quantitative NMR measurements. Pyrrolidine>piperidine>morpholine>diethylamine>dipropylamine>diisopropylaminiĪll of the products were identified by NMR, IR, GLC and elemental analyses and isomeric ratios The activity sequence of these secondary amine nucleophiles are attributed to both steric and With the most active pyrrolidine howwer, attack at the position This is because of the fact that reaction in polar solvents favors theįormation of the products with substituent para to chlorine, but the p-dichlorotetrafluorobenzene With pyrrolidine, only the ni- and o-dichlorotetrafluorobenzene reacted with the secondary amines toįorm the expected products. With chloropentafluorobenzene, only the para substituted products wereĭichlorotetrafluorobenzene was available as an isomeric mixtures of m:o:p=73 :IS :9. Prolonging reaction time or raising the reaction temperature caused the formation of the para disubstituted products in DMF. With pefluorobenzene monosubstituted products were obtained.

Reacted with pyrrolidine, piperidine, morpholine, diethylamine and dipropylamine in aprotic poiat Perfluorobenzene, chloropentafluorobenzene and dichlorotetrafluorobenzene were
Shanghai Insfiiiiie of Ordanic Chemistry, Academia Sinica, Shnn,nhoi Keactions with secondary a m i m nucleophiles


 0 kommentar(er)
0 kommentar(er)
